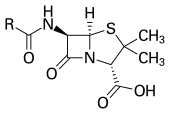
Antibiotics revolutionized medicine in the 20th century.
[9] Together with
vaccination, antibiotics have led to the near eradication of diseases such as
tuberculosis in the developed world. However, their effectiveness and easy access have also led to their
overuse,
[10][11][12] prompting bacteria to develop
resistance.
[2][13] This has led to widespread problems, so much as to prompt the
World Health Organization to classify antimicrobial resistance as a "serious threat [that] is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country"
Biological antibiotics derived from molds[edit]
Substances with antibiotic properties had been used for various purposes since ancient times.
Before the early 20th century, treatments for infections were based primarily on
medicinal folklore. Mixtures with antimicrobial properties that were used in treatments of infections were described over 2000 years ago.
[15] Many ancient cultures, including the
ancient Egyptians and
ancient Greeks, used specially selected
mold and plant materials and extracts to treat
infections.
[16][17] More recent observations made in the laboratory of antibiosis between microorganisms led to the discovery of natural antibacterials produced by microorganisms.
Louis Pasteur observed, "if we could intervene in the antagonism observed between some bacteria, it would offer perhaps the greatest hopes for therapeutics".
[18]
In 1895
Vincenzo Tiberio, Itallian physician, published a paper on the antibacterial power of some extracts of mold.
[21]
In 1897, doctoral student
Ernest Duchesne submitted a dissertation, "Contribution à l'étude de la concurrence vitale chez les micro-organismes: antagonisme entre les moisissures et les microbes" (Contribution to the study of vital competition in micro-organisms: antagonism between molds and microbes),
[22] the first known scholarly work to consider the therapeutic capabilities of molds resulting from their anti-microbial activity. In his thesis, Duchesne proposed that bacteria and molds engage in a perpetual battle for survival. Duchesne observed that
E. coli was eliminated by
Penicillium glaucum when they were both grown in the same culture. He also observed that when he
inoculated laboratory animals with lethal doses of
typhoid bacilli together with
Penicillium glaucum, the animals did not contract typhoid. Unfortunately Duchesne's army service after getting his degree prevented him from doing any further research.
[23] Duchesne died of
tuberculosis, a disease now treated by antibiotics.
[23]

Alexander Fleming was awarded a Nobel prize for his role in the discovery of penicillin
In 1928, Sir
Alexander Fleming identified
penicillin, a molecule produced by certain molds that kills or stops the growth of certain kinds of bacteria. Fleming was working on a culture of
disease-causing bacteria when he noticed the
spores of a green mold,
Penicillium chrysogenum, in one of his
culture plates. He observed that the presence of the mold killed or prevented the growth of the bacteria.
[24]Fleming postulated that the mold must secrete an antibacterial substance, which he named penicillin in 1928. Fleming believed that its antibacterial properties could be exploited for chemotherapy. He initially characterized some of its biological properties, and attempted to use a crude preparation to treat some infections, but he was unable to pursue its further development without the aid of trained chemists.
[25][26]
Ernst Chain,
Howard Florey and
Edward Abraham succeeded in purifying the first penicillin,
penicillin G, in 1942, but it did not become widely available outside the Allied military before 1945. Later,
Norman Heatley developed the back extraction technique for efficiently purifying penicillin in bulk. The chemical structure of penicillin was first proposed by Abraham in 1942
[27] and then later confirmed by
Dorothy Crowfoot Hodgkin in 1945. Purified penicillin displayed potent antibacterial activity against a wide range of bacteria and had low toxicity in humans. Furthermore, its activity was not inhibited by biological constituents such as pus, unlike the synthetic
sulfonamides. (see below) The discovery of such a powerful antibiotic was unprecedented, and the development of penicillin led to renewed interest in the search for antibiotic compounds with similar efficacy and safety.
[28] For their successful development of penicillin, which Fleming had accidentally discovered but could not develop himself, as a therapeutic drug, Chain and Florey shared the 1945
Nobel Prize in Medicine with Fleming.
Florey credited
Rene Dubos with pioneering the approach of deliberately and systematically searching for antibacterial compounds, which had led to the discovery of gramicidin and had revived Florey's research in penicillin.
[29] In 1939, coinciding with the start of
World War II, Dubos had reported the discovery of the first naturally derived antibiotic,
tyrothricin, a compound of 20%
gramicidin and 80%
tyrocidine, from
B. brevis. It was one of the first commercially manufactured antibiotics and was very effective in treating wounds and ulcers during World War II.
[29] Gramicidin, however, could not be used systemically because of toxicity. Tyrocidine also proved too toxic for systemic usage. Research results obtained during that period were not shared between the
Axis and the
Allied powers during World War II and limited access during the
Cold War.
[30]
Synthetic antibiotics derived from dyes[edit]
Synthetic antibiotic chemotherapy as a science and development of antibacterials began in Germany with
Paul Ehrlich in the late 1880s.
[31] Ehrlich noted certain dyes would color human, animal, or bacterial cells, whereas others did not. He then proposed the idea that it might be possible to create chemicals that would act as a selective drug that would bind to and kill bacteria without harming the human host. After screening hundreds of dyes against various organisms, in 1907, he discovered a medicinally useful drug, the first synthetic antibacterial
salvarsan[31][32][33] now called arsphenamine.
The era of antibacterial treatment began with the discoveries of arsenic-derived synthetic antibiotics by
Alfred Bertheim and Ehrlich in 1907.
[34][35] Ehrlich and Bertheim experimented with various chemicals derived from dyes to treat
trypanosomiasis in mice and
spirochaetainfection in rabbits. While their early compounds were too toxic, Ehrlich and
Sahachiro Hata, a Japanese bacteriologist working with Erlich in the quest for a drug to treat
syphilis, achieved success with the 606th compound in their series of experiments. In 1910 Ehrlich and Hata announced their discovery, which they called drug "606", at the Congress for Internal Medicine at
Wiesbaden.
[36] The
Hoechst company began to market the compound toward the end of 1910 under the name Salvarsan. This drug is now known as
arsphenamine.
[36] The drug was used to treat syphilis in the first half of the 20th century. In 1908, Ehrlich received the
Nobel Prize in Physiology or Medicine for his contributions to
immunology.
[37] Hata was nominated for the
Nobel Prize in Chemistry in 1911 and for the Nobel Prize in Physiology or Medicine in 1912 and 1913.
[38]
The first
sulfonamide and the first
systemically active antibacterial drug,
Prontosil, was developed by a research team led by
Gerhard Domagk in 1932 or 1933 at the
Bayer Laboratories of the
IG Farben conglomerate in Germany,
[35][39][33] for which Domagk received the 1939 Nobel Prize in Physiology or Medicine.
[40] Sulfanilamide, the active drug of Prontosil, was not patentable as it had already been in use in the dye industry for some years.
[39] Prontosil had a relatively broad effect against
Gram-positive cocci, but not against
enterobacteria. Research was stimulated apace by its success. The discovery and development of this sulfonamide
drug opened the era of antibacterials.
[41][42]
Medical uses[edit]
When the responsible pathogenic microorganism is already known or has been identified,
definitive therapy can be started. This will usually involve the use of a narrow-spectrum antibiotic. The choice of antibiotic given will also be based on its cost. Identification is critically important as it can reduce the cost and toxicity of the antibiotic therapy and also reduce the possibility of the emergence of antimicrobial resistance.
[44] To avoid surgery, antibiotics may be given for non-complicated acute
appendicitis.
[45]
Administration[edit]
There are different
routes of administration for antibiotic treatment. Antibiotics are usually
taken by mouth. In more severe cases, particularly deep-seated
systemic infections, antibiotics can be given
intravenously or by injection.
[2][44] Where the site of infection is easily accessed, antibiotics may be given
topically in the form of
eye drops onto the
conjunctiva for
conjunctivitis or
ear drops for ear infections and acute cases of
swimmer's ear. Topical use is also one of the treatment options for some skin conditions including
acneand
cellulitis.
[48] Advantages of topical application include achieving high and sustained concentration of antibiotic at the site of infection; reducing the potential for systemic absorption and toxicity, and total volumes of antibiotic required are reduced, thereby also reducing the risk of antibiotic misuse.
[49] Topical antibiotics applied over certain types of surgical wounds have been reported to reduce the risk of surgical site infections.
[50] However, there are certain general causes for concern with topical administration of antibiotics. Some systemic absorption of the antibiotic may occur; the quantity of antibiotic applied is difficult to accurately dose, and there is also the possibility of local
hypersensitivity reactions or
contact dermatitis occurring.
[49]
Side-effects[edit]

Health advocacy messages such as this one encourage patients to talk with their doctor about safety in using antibiotics.
Antibiotics are screened for any negative effects before their approval for clinical use, and are usually considered safe and well tolerated. However, some antibiotics have been associated with a wide extent of adverse
side effects ranging from mild to very severe depending on the type of antibiotic used, the microbes targeted, and the individual patient.
[51][52] Side effects may reflect the pharmacological or toxicological properties of the antibiotic or may involve hypersensitivity or
allergic reactions.
[5] Adverse effects range from fever and nausea to major allergic reactions, including
photodermatitis and
anaphylaxis.
[53] Safety profiles of newer drugs are often not as well established as for those that have a long history of use.
[51]
Correlation with obesity[edit]
Exposure to antibiotics early in life is associated with increased body mass in humans and mouse models.
[57] Early life is a critical period for the establishment of the
intestinal microbiota and for
metabolic development.
[58] Mice exposed to subtherapeutic antibiotic treatment (STAT)– with either penicillin,
vancomycin, or
chlortetracycline had altered composition of the gut microbiota as well as its metabolic capabilities.
[59]One study has reported that mice given low-dose penicillin (1 μg/g body weight) around birth and throughout the
weaning process had an increased body mass and fat mass, accelerated growth, and increased
hepatic expression of
genes involved in
adipogenesis, compared to control mice.
[60] In addition, penicillin in combination with a high-fat diet increased fasting
insulin levels in mice.
[60] However, it is unclear whether or not antibiotics cause
obesity in humans. Studies have found a correlation between early exposure of antibiotics (<6 months) and increased body mass (at 10 and 20 months).
[61] Another study found that the type of antibiotic exposure was also significant with the highest risk of being overweight in those given
macrolides compared to penicillin and
cephalosporin.
[62] Therefore, there is correlation between antibiotic exposure in early life and obesity in humans, but whether or not there is a causal relationship remains unclear. Although there is a correlation between antibiotic use in early life and obesity, the effect of antibiotics on obesity in humans needs to be weighed against the beneficial effects of clinically indicated treatment with antibiotics in infancy.
[58]
Interactions[edit]
Birth control pills[edit]
Well controlled studies on the effect of
oral contraceptive failure and antibiotics are very limited.
[63] The majority of studies indicate antibiotics do not interfere with
birth control pills,
[64] such as clinical studies that suggest the failure rate of contraceptive pills caused by antibiotics is very low (about 1%).
[65] Situations that may increase the risk of oral contraceptive failure include
non-compliance (missing taking the pill), vomiting or diarrhea. Gastrointestinal disorders or interpatient variability in oral contraceptive absorption affecting
ethinylestradiol serum levels in the blood.
[63] Women with
menstrual irregularities may be at higher risk of failure and should be advised to use
backup contraception during antibiotic treatment and for one week after its completion. If patient-specific risk factors for reduced oral contraceptive efficacy are suspected, backup contraception is recommended.
[63]
In cases where antibiotics have been suggested to affect the efficiency of birth control pills, such as for the broad-spectrum antibiotic
rifampicin, these cases may be due to an increase in the activities of hepatic liver enzymes' causing increased breakdown of the pill's active ingredients.
[64] Effects on the intestinal flora, which might result in reduced absorption of
estrogens in the colon, have also been suggested, but such suggestions have been inconclusive and controversial.
[66][67] Clinicians have recommended that extra contraceptive measures be applied during therapies using antibiotics that are suspected to interact with oral
contraceptives.
[64] More studies on the possible interactions between antibiotics and birth control pills (oral contraceptives) are required as well as careful assessment of patient-specific risk factors for potential oral contractive pill failure prior to dismissing the need for backup contraception.
[63]
Alcohol[edit]
Interactions between alcohol and certain antibiotics may occur and may cause side-effects and decreased effectiveness of antibiotic therapy.
[68][69] While moderate alcohol consumption is unlikely to interfere with many common antibiotics, there are specific types of antibiotics with which alcohol consumption may cause serious side-effects.
[70]Therefore, potential risks of side-effects and effectiveness depend on the type of antibiotic administered.
[71]
Antibiotics such as
metronidazole,
tinidazole,
cephamandole,
latamoxef,
cefoperazone,
cefmenoxime, and
furazolidone, cause a
disulfiram-like chemical reaction with alcohol by inhibiting its breakdown by
acetaldehyde dehydrogenase, which may result in vomiting, nausea, and shortness of breath.
[70] In addition, the efficacy of doxycycline and
erythromycinsuccinate may be reduced by alcohol consumption.
[72] Other effects of alcohol on antibiotic activity include altered activity of the liver enzymes that break down the antibiotic compound.
[73]
Pharmacodynamics[edit]
The successful outcome of antimicrobial therapy with antibacterial compounds depends on several factors. These include
host defense mechanisms, the location of infection, and the pharmacokinetic and pharmacodynamic properties of the antibacterial.
[74] A bactericidal activity of antibacterials may depend on the bacterial growth phase, and it often requires ongoing metabolic activity and division of bacterial cells.
[75] These findings are based on laboratory studies, and in clinical settings have also been shown to eliminate bacterial infection.
[74][76] Since the activity of antibacterials depends frequently on its concentration,
[77] in vitro characterization of antibacterial activity commonly includes the determination of the
minimum inhibitory concentration and minimum bactericidal concentration of an antibacterial.
[74][78] To predict clinical outcome, the antimicrobial activity of an antibacterial is usually combined with its
pharmacokinetic profile, and several pharmacological parameters are used as markers of drug efficacy.
[79]
Combination therapy[edit]
In important infectious diseases, including tuberculosis,
combination therapy (i.e., the concurrent application of two or more antibiotics) has been used to delay or prevent the emergence of resistance. In acute bacterial infections, antibiotics as part of combination therapy are prescribed for their
synergistic effects to improve treatment outcome as the combined effect of both antibiotics is better than their individual effect.
[80][81] Methicillin-resistant Staphylococcus aureus infections may be treated with a combination therapy of
fusidic acid and rifampicin.
[80] Antibiotics used in combination may also be antagonistic and the combined effects of the two antibiotics may be less than if the individual antibiotic was given as part of a
monotherapy.
[80] For example,
chloramphenicol and
tetracyclines are antagonists to
penicillins and
aminoglycosides. However, this can vary depending on the species of bacteria.
[82] In general, combinations of a bacteriostatic antibiotic and bactericidal antibiotic are antagonistic.
[80][81]
Classes[edit]

Molecular targets of antibiotics on the bacteria cell
Antibiotics are commonly classified based on their
mechanism of action,
chemical structure, or spectrum of activity. Most target bacterial functions or growth processes.
[31] Those that target the bacterial cell wall (
penicillins and
cephalosporins) or the cell membrane (
polymyxins), or interfere with essential bacterial enzymes (
rifamycins,
lipiarmycins,
quinolones, and
sulfonamides) have
bactericidal activities.
Protein synthesis inhibitors (
macrolides,
lincosamides and
tetracyclines) are usually
bacteriostatic (with the exception of bactericidal
aminoglycosides).
[83] Further categorization is based on their target specificity. "Narrow-spectrum" antibiotics target specific types of bacteria, such as
gram-negative or
gram-positive, whereas
broad-spectrum antibiotics affect a wide range of bacteria. Following a 40-year break in discovering new classes of antibacterial compounds, four new classes of antibiotics have been brought into clinical use in the late 2000s and early 2010s: cyclic
lipopeptides (such as
daptomycin),
glycylcyclines (such as
tigecycline),
oxazolidinones (such as
linezolid), and
lipiarmycins (such as
fidaxomicin).
[84][85]
Production[edit]
With advances in
medicinal chemistry, most modern antibacterials are
semisynthetic modifications of various natural compounds.
[86] These include, for example, the
beta-lactam antibiotics, which include the
penicillins (produced by fungi in the genus
Penicillium), the
cephalosporins, and the
carbapenems. Compounds that are still isolated from living organisms are the
aminoglycosides, whereas other antibacterials—for example, the
sulfonamides, the
quinolones, and the
oxazolidinones—are produced solely by
chemical synthesis.
[86] Many antibacterial compounds are relatively
small molecules with a
molecular weight of less than 1000
daltons.
[87]
Since the first pioneering efforts of
Howard Florey and
Chain in 1939, the importance of antibiotics, including antibacterials, to
medicine has led to intense research into producing antibacterials at large scales. Following screening of antibacterials against a wide range of bacteria, production of the active compounds is carried out using
fermentation, usually in strongly aerobic conditions.
[citation needed]
Resistance[edit]
The emergence of resistance of bacteria to antibiotics is a common phenomenon. Emergence of resistance often reflects
evolutionaryprocesses that take place during antibiotic therapy. The antibiotic treatment may
select for bacterial strains with physiologically or genetically enhanced capacity to survive high doses of antibiotics. Under certain conditions, it may result in preferential growth of resistant bacteria, while growth of susceptible bacteria is inhibited by the drug.
[88] For example, antibacterial selection for strains having previously acquired antibacterial-resistance genes was demonstrated in 1943 by the
Luria–Delbrück experiment.
[89] Antibiotics such as penicillin and erythromycin, which used to have a high efficacy against many bacterial species and strains, have become less effective, due to the increased resistance of many bacterial strains.
[90]
Resistance may take the form of biodegredation of pharmaceuticals, such as sulfamethazine-degrading soil bacteria introduced to sulfamethazine through medicated pig feces.
[91] The survival of bacteria often results from an inheritable resistance,
[92] but the growth of resistance to antibacterials also occurs through
horizontal gene transfer. Horizontal transfer is more likely to happen in locations of frequent antibiotic use.
[93]
Antibacterial resistance may impose a biological cost, thereby reducing
fitness of resistant strains, which can limit the spread of antibacterial-resistant bacteria, for example, in the absence of antibacterial compounds. Additional mutations, however, may compensate for this fitness cost and can aid the survival of these bacteria.
[94]
Paleontological data show that both antibiotics and antibiotic resistance are ancient compounds and mechanisms.
[95] Useful antibiotic targets are those for which mutations negatively impact bacterial reproduction or viability.
[96]
Several molecular mechanisms of antibacterial resistance exist. Intrinsic antibacterial resistance may be part of the genetic makeup of bacterial strains.
[97][98] For example, an antibiotic target may be absent from the bacterial
genome. Acquired resistance results from a mutation in the bacterial chromosome or the acquisition of extra-chromosomal DNA.
[97] Antibacterial-producing bacteria have evolved resistance mechanisms that have been shown to be similar to, and may have been transferred to, antibacterial-resistant strains.
[99][100] The spread of antibacterial resistance often occurs through vertical transmission of mutations during growth and by genetic recombination of DNA by
horizontal genetic exchange.
[92] For instance, antibacterial resistance genes can be exchanged between different bacterial strains or species via
plasmidsthat carry these resistance genes.
[92][101] Plasmids that carry several different resistance genes can confer resistance to multiple antibacterials.
[101] Cross-resistance to several antibacterials may also occur when a resistance mechanism encoded by a single gene conveys resistance to more than one antibacterial compound.
[101]
Antibacterial-resistant strains and species, sometimes referred to as "superbugs", now contribute to the emergence of diseases that were for a while well controlled. For example, emergent bacterial strains causing tuberculosis that are resistant to previously effective antibacterial treatments pose many therapeutic challenges. Every year, nearly half a million new cases of
multidrug-resistant tuberculosis (MDR-TB) are estimated to occur worldwide.
[102] For example,
NDM-1 is a newly identified enzyme conveying bacterial resistance to a broad range of
beta-lactam antibacterials.
[103] The United Kingdom's
Health Protection Agency has stated that "most isolates with NDM-1 enzyme are resistant to all standard intravenous antibiotics for treatment of severe infections."
[104] On 26 May 2016 an
E coli bacteria "
superbug" was identified in the
United States resistant to
colistin,
"the last line of defence" antibiotic.
[105][106]

This poster from the US Centers for Disease Control and Prevention "Get Smart" campaign, intended for use in doctors' offices and other healthcare facilities, warns that antibiotics do not work for viral illnesses such as the common cold.
Per
The ICU Book "The first rule of antibiotics is try not to use them, and the second rule is try not to use too many of them."
[107]Inappropriate antibiotic treatment and overuse of antibiotics have contributed to the emergence of antibiotic-resistant bacteria.
Self prescription of antibiotics is an example of misuse.
[108] Many antibiotics are frequently prescribed to treat symptoms or diseases that do not respond to antibiotics or that are likely to resolve without treatment. Also, incorrect or suboptimal antibiotics are prescribed for certain bacterial infections.
[51][108] The overuse of antibiotics, like penicillin and erythromycin, has been associated with emerging antibiotic resistance since the 1950s.
[90][109] Widespread usage of antibiotics in hospitals has also been associated with increases in bacterial strains and species that no longer respond to treatment with the most common antibiotics.
[109]
Common forms of antibiotic misuse include excessive use of
prophylactic antibiotics in travelers and failure of medical professionals to prescribe the correct dosage of antibiotics on the basis of the patient's weight and history of prior use. Other forms of misuse include failure to take the entire prescribed course of the antibiotic, incorrect dosage and administration, or failure to rest for sufficient recovery. Inappropriate antibiotic treatment, for example, is their prescription to treat viral infections such as the
common cold. One study on
respiratory tract infections found "physicians were more likely to prescribe antibiotics to patients who appeared to expect them".
[110]Multifactorial interventions aimed at both physicians and patients can reduce inappropriate prescription of antibiotics.
[111][112]
Several organizations concerned with antimicrobial resistance are lobbying to eliminate the unnecessary use of antibiotics.
[108] The issues of misuse and overuse of antibiotics have been addressed by the formation of the US Interagency Task Force on Antimicrobial Resistance. This task force aims to actively address antimicrobial resistance, and is coordinated by the US
Centers for Disease Control and Prevention, the
Food and Drug Administration (FDA), and the
National Institutes of Health (NIH), as well as other US agencies.
[113] An NGO campaign group is
Keep Antibiotics Working.
[114] In France, an "Antibiotics are not automatic" government campaign started in 2002 and led to a marked reduction of unnecessary antibiotic prescriptions, especially in children.
[115]
The emergence of antibiotic resistance has prompted restrictions on their use in the UK in 1970 (Swann report 1969), and the EU has banned the use of antibiotics as growth-promotional agents since 2003.
[116] Moreover, several organizations (including the World Health Organization, the
National Academy of Sciences, and the
U.S. Food and Drug Administration) have advocated restricting the amount of antibiotic use in food animal production.
[117] However, commonly there are delays in regulatory and legislative actions to limit the use of antibiotics, attributable partly to resistance against such regulation by industries using or selling antibiotics, and to the time required for research to test causal links between their use and resistance to them. Two federal bills (S.742
[118] and H.R. 2562
[119]) aimed at phasing out nontherapeutic use of antibiotics in US food animals were proposed, but have not passed.
[118][119] These bills were endorsed by public health and medical organizations, including the American Holistic Nurses' Association, the American Medical Association, and the American Public Health Association (APHA).
[120]
Despite pledges by food companies and restaurants to reduce or eliminate meat that comes from animals treated with antibiotics, the purchase of antibiotics for use on farm animals has been increasing every year.
[121]
There has been extensive use of antibiotics in animal husbandry. In the United States, the question of emergence of antibiotic-resistant bacterial strains due to
use of antibiotics in livestock was raised by the US
Food and Drug Administration (FDA) in 1977. In March 2012, the United States District Court for the Southern District of New York, ruling in an action brought by the
Natural Resources Defense Council and others, ordered the FDA to revoke approvals for the use of antibiotics in livestock, which violated FDA regulations.
[122]
Etymology[edit]
The term 'antibiosis', meaning "against life", was introduced by the French bacteriologist
Jean Paul Vuillemin as a descriptive name of the phenomenon exhibited by these early antibacterial drugs.
[31][123][124] Antibiosis was first described in 1877 in bacteria when Louis Pasteur and
Robert Koch observed that an airborne bacillus could inhibit the growth of
Bacillus anthracis.
[123][125] These drugs were later renamed antibiotics by
Selman Waksman, an American microbiologist, in 1942.
[31][123][126]
The term
antibiotic was first used in 1942 by
Selman Waksman and his collaborators in journal articles to describe any substance produced by a microorganism that is
antagonistic to the growth of other microorganisms in high dilution.
[123][126] This definition excluded substances that kill bacteria but that are not produced by microorganisms (such as
gastric juicesand
hydrogen peroxide). It also excluded
synthetic antibacterial compounds such as the
sulfonamides. In current usage, the term "antibiotic" is applied to any medication that kills bacteria or inhibits their growth, regardless of whether that medication is produced by a microorganism or not.
[127][128]
The term "antibiotic" derives from
anti + βιωτικός (
biōtikos), "fit for life, lively",
[129] which comes from βίωσις (
biōsis), "way of life",
[130] and that from βίος (
bios), "life".
[73][131] The term "antibacterial" derives from
Greek ἀντί (
anti), "against"
[132] + βακτήριον (
baktērion), diminutive of βακτηρία (
baktēria), "staff, cane",
[133] because the first ones to be discovered were rod-shaped.
[134]
Research[edit]
Alternatives[edit]
The increase in bacterial strains that are resistant to conventional antibacterial therapies together with decreasing number of new antibiotics currently being developed in the
drug pipeline has prompted the development of bacterial disease treatment strategies that are alternatives to conventional antibacterials.
[135][136] Non-compound approaches (that is, products other than classical antibacterial agents) that target bacteria or approaches that target the host including
phage therapy and
vaccines are also being investigated to combat the problem.
[137]
Resistance-modifying agents[edit]
One strategy to address bacterial drug resistance is the discovery and application of compounds that modify resistance to common antibacterials. Resistance modifying agents are capable of partly or completely suppressing bacterial resistance mechanisms.
[138] For example, some resistance-modifying agents may inhibit multidrug resistance mechanisms, such as
drug efflux from the cell, thus increasing the susceptibility of bacteria to an antibacterial.
[138][139] Targets include:
Metabolic stimuli such as sugar can help eradicate a certain type of antibiotic-tolerant bacteria by keeping their metabolism active.
[141]
Vaccines[edit]
Vaccines rely on
immune modulation or augmentation. Vaccination either excites or reinforces the immune competence of a host to ward off infection, leading to the activation of
macrophages, the production of
antibodies,
inflammation, and other classic immune reactions. Antibacterial vaccines have been responsible for a drastic reduction in global bacterial diseases.
[142] Vaccines made from attenuated whole cells or lysates have been replaced largely by less reactogenic, cell-free vaccines consisting of purified components, including capsular polysaccharides and their conjugates, to protein carriers, as well as inactivated toxins (toxoids) and proteins.
[143]
Phage therapy[edit]

Phage injecting its genome into bacterial cell
Phage therapy is another method for treating antibiotic-resistant strains of bacteria. Phage therapy infects pathogenic bacteria with their own viruses,
bacteriophages and their host ranges are extremely specific for certain bacteria, thus they do not disturb the host organism and intestinal microflora unlike antibiotics.
[144] Bacteriophages, also known simply as phages, infect and can kill bacteria and affect bacterial growth primarily during lytic cycles.
[144][145] Phages insert their DNA into the bacterium, where it is transcribed and used to make new phages, after which the cell will lyse, releasing new phage able to infect and destroy further bacteria of the same strain.
[145] The high specificity of phage protects "good" bacteria from destruction. However, some disadvantages to use of bacteriophages also exist. Bacteriophages may harbour virulence factors or toxic genes in their genomes and identification of genes with similarity to known virulence factors or toxins by genomic sequencing may be prudent prior to use. In addition, the oral and IV administration of phages for the eradication of bacterial infections poses a much higher safety risk than topical application, and there is the additional concern of uncertain immune responses to these large antigenic cocktails. There are considerable regulatory hurdles that must be cleared for such therapies.
[144] The use of bacteriophages as a replacement for antimicrobial agents against MDR pathogens no longer respond to conventional antibiotics remains an attractive option despite numerous challenges.
[144][146]
Phytochemicals[edit]
Plants are an important source of antimicrobial compounds and traditional healers have long used plants to prevent or cure infectious diseases.
[147][148] There is a recent renewed interest into the use of natural products for the identification of new members of the 'antibiotic-ome' (defined as natural products with antibiotic activity), and their application in antibacterial drug discovery in
the genomics era.
[135][149] Phytochemicals are the active biological component of plants and some phytochemicals including
tannins,
alkaloids,
terpenoids and
flavonoids possess antimicrobial activity.
[147][150][151] Some
antioxidant dietary supplements also contain phytochemicals (
polyphenols), such as
grape seed extract, and demonstrate
in vitro anti-bacterial properties.
[152][153][154] Phytochemicals are able to inhibit peptidoglycan synthesis, damage microbial membrane structures, modify bacterial membrane surface hydrophobicity and also modulate quorum-sensing.
[150] With increasing antibiotic resistance in recent years, the potential of new plant-derived antibiotics is under investigation.
[149]
Development of new antibiotics[edit]
In April 2013, the
Infectious Disease Society of America (IDSA) reported that the weak antibiotic pipeline does not match bacteria's increasing ability to develop resistance. Since 2009, only 2 new antibiotics were approved in the United States. The number of new antibiotics approved for marketing per year declines continuously. The report identified seven antibiotics against the
Gram-negative bacilli (GNB) currently in
phase 2 or
phase 3 clinical trials. However, these drugs do not address the entire spectrum of resistance of GNB.
[155][156] Some of these antibiotics are combination of existent treatments:
[citation needed]
Streptomyces research is expected to provide new antibiotics, including treatment against
MRSA and infections resistant to commonly used medication. Efforts of
John Innes Centreand universities in the UK, supported by BBSRC, resulted in the creation of spin-out companies, for example Novacta Biosystems, which has designed the type-b
lantibiotic-based compound NVB302 (in phase 1) to treat
Clostridium difficile infections.
[158][159] Possible improvements include clarification of clinical trial regulations by FDA. Furthermore, appropriate economic incentives could persuade pharmaceutical companies to invest in this endeavor.
[156] In the US, the
Antibiotic Development to Advance Patient Treatment(ADAPT) Act was introduced with the aim of fast tracking the drug development of antibiotics to combat the growing threat of 'superbugs'. Under this Act, FDA can approve antibiotics and antifungals treating life-threatening infections based on smaller clinical trials. The
CDC will monitor the use of antibiotics and the emerging resistance, and publish the data. The FDA antibiotics labeling process, 'Susceptibility Test Interpretive Criteria for Microbial Organisms' or 'breakpoints', will provide accurate data to healthcare professionals.
[160][161]According to Allan Coukell, senior director for health programs at The Pew Charitable Trusts, "By allowing drug developers to rely on smaller datasets, and clarifying FDA's authority to tolerate a higher level of uncertainty for these drugs when making a risk/benefit calculation, ADAPT would make the clinical trials more feasible."
Biological antibiotics derived from molds[edit]
Substances with antibiotic properties had been used for various purposes since ancient times.
Before the early 20th century, treatments for infections were based primarily on
medicinal folklore. Mixtures with antimicrobial properties that were used in treatments of infections were described over 2000 years ago.
[15] Many ancient cultures, including the
ancient Egyptians and
ancient Greeks, used specially selected
mold and plant materials and extracts to treat
infections.
[16][17] More recent observations made in the laboratory of antibiosis between microorganisms led to the discovery of natural antibacterials produced by microorganisms.
Louis Pasteur observed, "if we could intervene in the antagonism observed between some bacteria, it would offer perhaps the greatest hopes for therapeutics".
[18]
In 1895
Vincenzo Tiberio, Itallian physician, published a paper on the antibacterial power of some extracts of mold.
[21]
In 1897, doctoral student
Ernest Duchesne submitted a dissertation, "Contribution à l'étude de la concurrence vitale chez les micro-organismes: antagonisme entre les moisissures et les microbes" (Contribution to the study of vital competition in micro-organisms: antagonism between molds and microbes),
[22] the first known scholarly work to consider the therapeutic capabilities of molds resulting from their anti-microbial activity. In his thesis, Duchesne proposed that bacteria and molds engage in a perpetual battle for survival. Duchesne observed that
E. coli was eliminated by
Penicillium glaucum when they were both grown in the same culture. He also observed that when he
inoculated laboratory animals with lethal doses of
typhoid bacilli together with
Penicillium glaucum, the animals did not contract typhoid. Unfortunately Duchesne's army service after getting his degree prevented him from doing any further research.
[23] Duchesne died of
tuberculosis, a disease now treated by antibiotics.
[23]

Alexander Fleming was awarded a Nobel prize for his role in the discovery of penicillin
In 1928, Sir
Alexander Fleming identified
penicillin, a molecule produced by certain molds that kills or stops the growth of certain kinds of bacteria. Fleming was working on a culture of
disease-causing bacteria when he noticed the
spores of a green mold,
Penicillium chrysogenum, in one of his
culture plates. He observed that the presence of the mold killed or prevented the growth of the bacteria.
[24]Fleming postulated that the mold must secrete an antibacterial substance, which he named penicillin in 1928. Fleming believed that its antibacterial properties could be exploited for chemotherapy. He initially characterized some of its biological properties, and attempted to use a crude preparation to treat some infections, but he was unable to pursue its further development without the aid of trained chemists.
[25][26]
Ernst Chain,
Howard Florey and
Edward Abraham succeeded in purifying the first penicillin,
penicillin G, in 1942, but it did not become widely available outside the Allied military before 1945. Later,
Norman Heatley developed the back extraction technique for efficiently purifying penicillin in bulk. The chemical structure of penicillin was first proposed by Abraham in 1942
[27] and then later confirmed by
Dorothy Crowfoot Hodgkin in 1945. Purified penicillin displayed potent antibacterial activity against a wide range of bacteria and had low toxicity in humans. Furthermore, its activity was not inhibited by biological constituents such as pus, unlike the synthetic
sulfonamides. (see below) The discovery of such a powerful antibiotic was unprecedented, and the development of penicillin led to renewed interest in the search for antibiotic compounds with similar efficacy and safety.
[28] For their successful development of penicillin, which Fleming had accidentally discovered but could not develop himself, as a therapeutic drug, Chain and Florey shared the 1945
Nobel Prize in Medicine with Fleming.
Florey credited
Rene Dubos with pioneering the approach of deliberately and systematically searching for antibacterial compounds, which had led to the discovery of gramicidin and had revived Florey's research in penicillin.
[29] In 1939, coinciding with the start of
World War II, Dubos had reported the discovery of the first naturally derived antibiotic,
tyrothricin, a compound of 20%
gramicidin and 80%
tyrocidine, from
B. brevis. It was one of the first commercially manufactured antibiotics and was very effective in treating wounds and ulcers during World War II.
[29] Gramicidin, however, could not be used systemically because of toxicity. Tyrocidine also proved too toxic for systemic usage. Research results obtained during that period were not shared between the
Axis and the
Allied powers during World War II and limited access during the
Cold War.
[30]
Synthetic antibiotics derived from dyes[edit]
Synthetic antibiotic chemotherapy as a science and development of antibacterials began in Germany with
Paul Ehrlich in the late 1880s.
[31] Ehrlich noted certain dyes would color human, animal, or bacterial cells, whereas others did not. He then proposed the idea that it might be possible to create chemicals that would act as a selective drug that would bind to and kill bacteria without harming the human host. After screening hundreds of dyes against various organisms, in 1907, he discovered a medicinally useful drug, the first synthetic antibacterial
salvarsan[31][32][33] now called arsphenamine.
The era of antibacterial treatment began with the discoveries of arsenic-derived synthetic antibiotics by
Alfred Bertheim and Ehrlich in 1907.
[34][35] Ehrlich and Bertheim experimented with various chemicals derived from dyes to treat
trypanosomiasis in mice and
spirochaetainfection in rabbits. While their early compounds were too toxic, Ehrlich and
Sahachiro Hata, a Japanese bacteriologist working with Erlich in the quest for a drug to treat
syphilis, achieved success with the 606th compound in their series of experiments. In 1910 Ehrlich and Hata announced their discovery, which they called drug "606", at the Congress for Internal Medicine at
Wiesbaden.
[36] The
Hoechst company began to market the compound toward the end of 1910 under the name Salvarsan. This drug is now known as
arsphenamine.
[36] The drug was used to treat syphilis in the first half of the 20th century. In 1908, Ehrlich received the
Nobel Prize in Physiology or Medicine for his contributions to
immunology.
[37] Hata was nominated for the
Nobel Prize in Chemistry in 1911 and for the Nobel Prize in Physiology or Medicine in 1912 and 1913.
[38]
The first
sulfonamide and the first
systemically active antibacterial drug,
Prontosil, was developed by a research team led by
Gerhard Domagk in 1932 or 1933 at the
Bayer Laboratories of the
IG Farben conglomerate in Germany,
[35][39][33] for which Domagk received the 1939 Nobel Prize in Physiology or Medicine.
[40] Sulfanilamide, the active drug of Prontosil, was not patentable as it had already been in use in the dye industry for some years.
[39] Prontosil had a relatively broad effect against
Gram-positive cocci, but not against
enterobacteria. Research was stimulated apace by its success. The discovery and development of this sulfonamide
drug opened the era of antibacterials.
[41][42]
Medical uses[edit]
When the responsible pathogenic microorganism is already known or has been identified,
definitive therapy can be started. This will usually involve the use of a narrow-spectrum antibiotic. The choice of antibiotic given will also be based on its cost. Identification is critically important as it can reduce the cost and toxicity of the antibiotic therapy and also reduce the possibility of the emergence of antimicrobial resistance.
[44] To avoid surgery, antibiotics may be given for non-complicated acute
appendicitis.
[45]
Administration[edit]
There are different
routes of administration for antibiotic treatment. Antibiotics are usually
taken by mouth. In more severe cases, particularly deep-seated
systemic infections, antibiotics can be given
intravenously or by injection.
[2][44] Where the site of infection is easily accessed, antibiotics may be given
topically in the form of
eye drops onto the
conjunctiva for
conjunctivitis or
ear drops for ear infections and acute cases of
swimmer's ear. Topical use is also one of the treatment options for some skin conditions including
acneand
cellulitis.
[48] Advantages of topical application include achieving high and sustained concentration of antibiotic at the site of infection; reducing the potential for systemic absorption and toxicity, and total volumes of antibiotic required are reduced, thereby also reducing the risk of antibiotic misuse.
[49] Topical antibiotics applied over certain types of surgical wounds have been reported to reduce the risk of surgical site infections.
[50] However, there are certain general causes for concern with topical administration of antibiotics. Some systemic absorption of the antibiotic may occur; the quantity of antibiotic applied is difficult to accurately dose, and there is also the possibility of local
hypersensitivity reactions or
contact dermatitis occurring.
[49]
Side-effects[edit]

Health advocacy messages such as this one encourage patients to talk with their doctor about safety in using antibiotics.
Antibiotics are screened for any negative effects before their approval for clinical use, and are usually considered safe and well tolerated. However, some antibiotics have been associated with a wide extent of adverse
side effects ranging from mild to very severe depending on the type of antibiotic used, the microbes targeted, and the individual patient.
[51][52] Side effects may reflect the pharmacological or toxicological properties of the antibiotic or may involve hypersensitivity or
allergic reactions.
[5] Adverse effects range from fever and nausea to major allergic reactions, including
photodermatitis and
anaphylaxis.
[53] Safety profiles of newer drugs are often not as well established as for those that have a long history of use.
[51]
Correlation with obesity[edit]
Exposure to antibiotics early in life is associated with increased body mass in humans and mouse models.
[57] Early life is a critical period for the establishment of the
intestinal microbiota and for
metabolic development.
[58] Mice exposed to subtherapeutic antibiotic treatment (STAT)– with either penicillin,
vancomycin, or
chlortetracycline had altered composition of the gut microbiota as well as its metabolic capabilities.
[59]One study has reported that mice given low-dose penicillin (1 μg/g body weight) around birth and throughout the
weaning process had an increased body mass and fat mass, accelerated growth, and increased
hepatic expression of
genes involved in
adipogenesis, compared to control mice.
[60] In addition, penicillin in combination with a high-fat diet increased fasting
insulin levels in mice.
[60] However, it is unclear whether or not antibiotics cause
obesity in humans. Studies have found a correlation between early exposure of antibiotics (<6 months) and increased body mass (at 10 and 20 months).
[61] Another study found that the type of antibiotic exposure was also significant with the highest risk of being overweight in those given
macrolides compared to penicillin and
cephalosporin.
[62] Therefore, there is correlation between antibiotic exposure in early life and obesity in humans, but whether or not there is a causal relationship remains unclear. Although there is a correlation between antibiotic use in early life and obesity, the effect of antibiotics on obesity in humans needs to be weighed against the beneficial effects of clinically indicated treatment with antibiotics in infancy.
[58]
Interactions[edit]
Birth control pills[edit]
Well controlled studies on the effect of
oral contraceptive failure and antibiotics are very limited.
[63] The majority of studies indicate antibiotics do not interfere with
birth control pills,
[64] such as clinical studies that suggest the failure rate of contraceptive pills caused by antibiotics is very low (about 1%).
[65] Situations that may increase the risk of oral contraceptive failure include
non-compliance (missing taking the pill), vomiting or diarrhea. Gastrointestinal disorders or interpatient variability in oral contraceptive absorption affecting
ethinylestradiol serum levels in the blood.
[63] Women with
menstrual irregularities may be at higher risk of failure and should be advised to use
backup contraception during antibiotic treatment and for one week after its completion. If patient-specific risk factors for reduced oral contraceptive efficacy are suspected, backup contraception is recommended.
[63]
In cases where antibiotics have been suggested to affect the efficiency of birth control pills, such as for the broad-spectrum antibiotic
rifampicin, these cases may be due to an increase in the activities of hepatic liver enzymes' causing increased breakdown of the pill's active ingredients.
[64] Effects on the intestinal flora, which might result in reduced absorption of
estrogens in the colon, have also been suggested, but such suggestions have been inconclusive and controversial.
[66][67] Clinicians have recommended that extra contraceptive measures be applied during therapies using antibiotics that are suspected to interact with oral
contraceptives.
[64] More studies on the possible interactions between antibiotics and birth control pills (oral contraceptives) are required as well as careful assessment of patient-specific risk factors for potential oral contractive pill failure prior to dismissing the need for backup contraception.
[63]
Alcohol[edit]
Interactions between alcohol and certain antibiotics may occur and may cause side-effects and decreased effectiveness of antibiotic therapy.
[68][69] While moderate alcohol consumption is unlikely to interfere with many common antibiotics, there are specific types of antibiotics with which alcohol consumption may cause serious side-effects.
[70]Therefore, potential risks of side-effects and effectiveness depend on the type of antibiotic administered.
[71]
Antibiotics such as
metronidazole,
tinidazole,
cephamandole,
latamoxef,
cefoperazone,
cefmenoxime, and
furazolidone, cause a
disulfiram-like chemical reaction with alcohol by inhibiting its breakdown by
acetaldehyde dehydrogenase, which may result in vomiting, nausea, and shortness of breath.
[70] In addition, the efficacy of doxycycline and
erythromycinsuccinate may be reduced by alcohol consumption.
[72] Other effects of alcohol on antibiotic activity include altered activity of the liver enzymes that break down the antibiotic compound.
[73]
Pharmacodynamics[edit]
The successful outcome of antimicrobial therapy with antibacterial compounds depends on several factors. These include
host defense mechanisms, the location of infection, and the pharmacokinetic and pharmacodynamic properties of the antibacterial.
[74] A bactericidal activity of antibacterials may depend on the bacterial growth phase, and it often requires ongoing metabolic activity and division of bacterial cells.
[75] These findings are based on laboratory studies, and in clinical settings have also been shown to eliminate bacterial infection.
[74][76] Since the activity of antibacterials depends frequently on its concentration,
[77] in vitro characterization of antibacterial activity commonly includes the determination of the
minimum inhibitory concentration and minimum bactericidal concentration of an antibacterial.
[74][78] To predict clinical outcome, the antimicrobial activity of an antibacterial is usually combined with its
pharmacokinetic profile, and several pharmacological parameters are used as markers of drug efficacy.
[79]
Combination therapy[edit]
In important infectious diseases, including tuberculosis,
combination therapy (i.e., the concurrent application of two or more antibiotics) has been used to delay or prevent the emergence of resistance. In acute bacterial infections, antibiotics as part of combination therapy are prescribed for their
synergistic effects to improve treatment outcome as the combined effect of both antibiotics is better than their individual effect.
[80][81] Methicillin-resistant Staphylococcus aureus infections may be treated with a combination therapy of
fusidic acid and rifampicin.
[80] Antibiotics used in combination may also be antagonistic and the combined effects of the two antibiotics may be less than if the individual antibiotic was given as part of a
monotherapy.
[80] For example,
chloramphenicol and
tetracyclines are antagonists to
penicillins and
aminoglycosides. However, this can vary depending on the species of bacteria.
[82] In general, combinations of a bacteriostatic antibiotic and bactericidal antibiotic are antagonistic.
[80][81]
Classes[edit]

Molecular targets of antibiotics on the bacteria cell
Antibiotics are commonly classified based on their
mechanism of action,
chemical structure, or spectrum of activity. Most target bacterial functions or growth processes.
[31] Those that target the bacterial cell wall (
penicillins and
cephalosporins) or the cell membrane (
polymyxins), or interfere with essential bacterial enzymes (
rifamycins,
lipiarmycins,
quinolones, and
sulfonamides) have
bactericidal activities.
Protein synthesis inhibitors (
macrolides,
lincosamides and
tetracyclines) are usually
bacteriostatic (with the exception of bactericidal
aminoglycosides).
[83] Further categorization is based on their target specificity. "Narrow-spectrum" antibiotics target specific types of bacteria, such as
gram-negative or
gram-positive, whereas
broad-spectrum antibiotics affect a wide range of bacteria. Following a 40-year break in discovering new classes of antibacterial compounds, four new classes of antibiotics have been brought into clinical use in the late 2000s and early 2010s: cyclic
lipopeptides (such as
daptomycin),
glycylcyclines (such as
tigecycline),
oxazolidinones (such as
linezolid), and
lipiarmycins (such as
fidaxomicin).
[84][85]
Production[edit]
With advances in
medicinal chemistry, most modern antibacterials are
semisynthetic modifications of various natural compounds.
[86] These include, for example, the
beta-lactam antibiotics, which include the
penicillins (produced by fungi in the genus
Penicillium), the
cephalosporins, and the
carbapenems. Compounds that are still isolated from living organisms are the
aminoglycosides, whereas other antibacterials—for example, the
sulfonamides, the
quinolones, and the
oxazolidinones—are produced solely by
chemical synthesis.
[86] Many antibacterial compounds are relatively
small molecules with a
molecular weight of less than 1000
daltons.
[87]
Since the first pioneering efforts of
Howard Florey and
Chain in 1939, the importance of antibiotics, including antibacterials, to
medicine has led to intense research into producing antibacterials at large scales. Following screening of antibacterials against a wide range of bacteria, production of the active compounds is carried out using
fermentation, usually in strongly aerobic conditions.
[citation needed]
Resistance[edit]
The emergence of resistance of bacteria to antibiotics is a common phenomenon. Emergence of resistance often reflects
evolutionaryprocesses that take place during antibiotic therapy. The antibiotic treatment may
select for bacterial strains with physiologically or genetically enhanced capacity to survive high doses of antibiotics. Under certain conditions, it may result in preferential growth of resistant bacteria, while growth of susceptible bacteria is inhibited by the drug.
[88] For example, antibacterial selection for strains having previously acquired antibacterial-resistance genes was demonstrated in 1943 by the
Luria–Delbrück experiment.
[89] Antibiotics such as penicillin and erythromycin, which used to have a high efficacy against many bacterial species and strains, have become less effective, due to the increased resistance of many bacterial strains.
[90]
Resistance may take the form of biodegredation of pharmaceuticals, such as sulfamethazine-degrading soil bacteria introduced to sulfamethazine through medicated pig feces.
[91] The survival of bacteria often results from an inheritable resistance,
[92] but the growth of resistance to antibacterials also occurs through
horizontal gene transfer. Horizontal transfer is more likely to happen in locations of frequent antibiotic use.
[93]
Antibacterial resistance may impose a biological cost, thereby reducing
fitness of resistant strains, which can limit the spread of antibacterial-resistant bacteria, for example, in the absence of antibacterial compounds. Additional mutations, however, may compensate for this fitness cost and can aid the survival of these bacteria.
[94]
Paleontological data show that both antibiotics and antibiotic resistance are ancient compounds and mechanisms.
[95] Useful antibiotic targets are those for which mutations negatively impact bacterial reproduction or viability.
[96]
Several molecular mechanisms of antibacterial resistance exist. Intrinsic antibacterial resistance may be part of the genetic makeup of bacterial strains.
[97][98] For example, an antibiotic target may be absent from the bacterial
genome. Acquired resistance results from a mutation in the bacterial chromosome or the acquisition of extra-chromosomal DNA.
[97] Antibacterial-producing bacteria have evolved resistance mechanisms that have been shown to be similar to, and may have been transferred to, antibacterial-resistant strains.
[99][100] The spread of antibacterial resistance often occurs through vertical transmission of mutations during growth and by genetic recombination of DNA by
horizontal genetic exchange.
[92] For instance, antibacterial resistance genes can be exchanged between different bacterial strains or species via
plasmidsthat carry these resistance genes.
[92][101] Plasmids that carry several different resistance genes can confer resistance to multiple antibacterials.
[101] Cross-resistance to several antibacterials may also occur when a resistance mechanism encoded by a single gene conveys resistance to more than one antibacterial compound.
[101]
Antibacterial-resistant strains and species, sometimes referred to as "superbugs", now contribute to the emergence of diseases that were for a while well controlled. For example, emergent bacterial strains causing tuberculosis that are resistant to previously effective antibacterial treatments pose many therapeutic challenges. Every year, nearly half a million new cases of
multidrug-resistant tuberculosis (MDR-TB) are estimated to occur worldwide.
[102] For example,
NDM-1 is a newly identified enzyme conveying bacterial resistance to a broad range of
beta-lactam antibacterials.
[103] The United Kingdom's
Health Protection Agency has stated that "most isolates with NDM-1 enzyme are resistant to all standard intravenous antibiotics for treatment of severe infections."
[104] On 26 May 2016 an
E coli bacteria "
superbug" was identified in the
United States resistant to
colistin,
"the last line of defence" antibiotic.
[105][106]

This poster from the US Centers for Disease Control and Prevention "Get Smart" campaign, intended for use in doctors' offices and other healthcare facilities, warns that antibiotics do not work for viral illnesses such as the common cold.
Per
The ICU Book "The first rule of antibiotics is try not to use them, and the second rule is try not to use too many of them."
[107]Inappropriate antibiotic treatment and overuse of antibiotics have contributed to the emergence of antibiotic-resistant bacteria.
Self prescription of antibiotics is an example of misuse.
[108] Many antibiotics are frequently prescribed to treat symptoms or diseases that do not respond to antibiotics or that are likely to resolve without treatment. Also, incorrect or suboptimal antibiotics are prescribed for certain bacterial infections.
[51][108] The overuse of antibiotics, like penicillin and erythromycin, has been associated with emerging antibiotic resistance since the 1950s.
[90][109] Widespread usage of antibiotics in hospitals has also been associated with increases in bacterial strains and species that no longer respond to treatment with the most common antibiotics.
[109]
Common forms of antibiotic misuse include excessive use of
prophylactic antibiotics in travelers and failure of medical professionals to prescribe the correct dosage of antibiotics on the basis of the patient's weight and history of prior use. Other forms of misuse include failure to take the entire prescribed course of the antibiotic, incorrect dosage and administration, or failure to rest for sufficient recovery. Inappropriate antibiotic treatment, for example, is their prescription to treat viral infections such as the
common cold. One study on
respiratory tract infections found "physicians were more likely to prescribe antibiotics to patients who appeared to expect them".
[110]Multifactorial interventions aimed at both physicians and patients can reduce inappropriate prescription of antibiotics.
[111][112]
Several organizations concerned with antimicrobial resistance are lobbying to eliminate the unnecessary use of antibiotics.
[108] The issues of misuse and overuse of antibiotics have been addressed by the formation of the US Interagency Task Force on Antimicrobial Resistance. This task force aims to actively address antimicrobial resistance, and is coordinated by the US
Centers for Disease Control and Prevention, the
Food and Drug Administration (FDA), and the
National Institutes of Health (NIH), as well as other US agencies.
[113] An NGO campaign group is
Keep Antibiotics Working.
[114] In France, an "Antibiotics are not automatic" government campaign started in 2002 and led to a marked reduction of unnecessary antibiotic prescriptions, especially in children.
[115]
The emergence of antibiotic resistance has prompted restrictions on their use in the UK in 1970 (Swann report 1969), and the EU has banned the use of antibiotics as growth-promotional agents since 2003.
[116] Moreover, several organizations (including the World Health Organization, the
National Academy of Sciences, and the
U.S. Food and Drug Administration) have advocated restricting the amount of antibiotic use in food animal production.
[117] However, commonly there are delays in regulatory and legislative actions to limit the use of antibiotics, attributable partly to resistance against such regulation by industries using or selling antibiotics, and to the time required for research to test causal links between their use and resistance to them. Two federal bills (S.742
[118] and H.R. 2562
[119]) aimed at phasing out nontherapeutic use of antibiotics in US food animals were proposed, but have not passed.
[118][119] These bills were endorsed by public health and medical organizations, including the American Holistic Nurses' Association, the American Medical Association, and the American Public Health Association (APHA).
[120]
Despite pledges by food companies and restaurants to reduce or eliminate meat that comes from animals treated with antibiotics, the purchase of antibiotics for use on farm animals has been increasing every year.
[121]
There has been extensive use of antibiotics in animal husbandry. In the United States, the question of emergence of antibiotic-resistant bacterial strains due to
use of antibiotics in livestock was raised by the US
Food and Drug Administration (FDA) in 1977. In March 2012, the United States District Court for the Southern District of New York, ruling in an action brought by the
Natural Resources Defense Council and others, ordered the FDA to revoke approvals for the use of antibiotics in livestock, which violated FDA regulations.
[122]
Etymology[edit]
The term 'antibiosis', meaning "against life", was introduced by the French bacteriologist
Jean Paul Vuillemin as a descriptive name of the phenomenon exhibited by these early antibacterial drugs.
[31][123][124] Antibiosis was first described in 1877 in bacteria when Louis Pasteur and
Robert Koch observed that an airborne bacillus could inhibit the growth of
Bacillus anthracis.
[123][125] These drugs were later renamed antibiotics by
Selman Waksman, an American microbiologist, in 1942.
[31][123][126]
The term
antibiotic was first used in 1942 by
Selman Waksman and his collaborators in journal articles to describe any substance produced by a microorganism that is
antagonistic to the growth of other microorganisms in high dilution.
[123][126] This definition excluded substances that kill bacteria but that are not produced by microorganisms (such as
gastric juicesand
hydrogen peroxide). It also excluded
synthetic antibacterial compounds such as the
sulfonamides. In current usage, the term "antibiotic" is applied to any medication that kills bacteria or inhibits their growth, regardless of whether that medication is produced by a microorganism or not.
[127][128]
The term "antibiotic" derives from
anti + βιωτικός (
biōtikos), "fit for life, lively",
[129] which comes from βίωσις (
biōsis), "way of life",
[130] and that from βίος (
bios), "life".
[73][131] The term "antibacterial" derives from
Greek ἀντί (
anti), "against"
[132] + βακτήριον (
baktērion), diminutive of βακτηρία (
baktēria), "staff, cane",
[133] because the first ones to be discovered were rod-shaped.
[134]
Research[edit]
Alternatives[edit]
The increase in bacterial strains that are resistant to conventional antibacterial therapies together with decreasing number of new antibiotics currently being developed in the
drug pipeline has prompted the development of bacterial disease treatment strategies that are alternatives to conventional antibacterials.
[135][136] Non-compound approaches (that is, products other than classical antibacterial agents) that target bacteria or approaches that target the host including
phage therapy and
vaccines are also being investigated to combat the problem.
[137]
Resistance-modifying agents[edit]
One strategy to address bacterial drug resistance is the discovery and application of compounds that modify resistance to common antibacterials. Resistance modifying agents are capable of partly or completely suppressing bacterial resistance mechanisms.
[138] For example, some resistance-modifying agents may inhibit multidrug resistance mechanisms, such as
drug efflux from the cell, thus increasing the susceptibility of bacteria to an antibacterial.
[138][139] Targets include:
Metabolic stimuli such as sugar can help eradicate a certain type of antibiotic-tolerant bacteria by keeping their metabolism active.
[141]
Vaccines[edit]
Vaccines rely on
immune modulation or augmentation. Vaccination either excites or reinforces the immune competence of a host to ward off infection, leading to the activation of
macrophages, the production of
antibodies,
inflammation, and other classic immune reactions. Antibacterial vaccines have been responsible for a drastic reduction in global bacterial diseases.
[142] Vaccines made from attenuated whole cells or lysates have been replaced largely by less reactogenic, cell-free vaccines consisting of purified components, including capsular polysaccharides and their conjugates, to protein carriers, as well as inactivated toxins (toxoids) and proteins.
[143]
Phage therapy[edit]

Phage injecting its genome into bacterial cell
Phage therapy is another method for treating antibiotic-resistant strains of bacteria. Phage therapy infects pathogenic bacteria with their own viruses,
bacteriophages and their host ranges are extremely specific for certain bacteria, thus they do not disturb the host organism and intestinal microflora unlike antibiotics.
[144] Bacteriophages, also known simply as phages, infect and can kill bacteria and affect bacterial growth primarily during lytic cycles.
[144][145] Phages insert their DNA into the bacterium, where it is transcribed and used to make new phages, after which the cell will lyse, releasing new phage able to infect and destroy further bacteria of the same strain.
[145] The high specificity of phage protects "good" bacteria from destruction. However, some disadvantages to use of bacteriophages also exist. Bacteriophages may harbour virulence factors or toxic genes in their genomes and identification of genes with similarity to known virulence factors or toxins by genomic sequencing may be prudent prior to use. In addition, the oral and IV administration of phages for the eradication of bacterial infections poses a much higher safety risk than topical application, and there is the additional concern of uncertain immune responses to these large antigenic cocktails. There are considerable regulatory hurdles that must be cleared for such therapies.
[144] The use of bacteriophages as a replacement for antimicrobial agents against MDR pathogens no longer respond to conventional antibiotics remains an attractive option despite numerous challenges.
[144][146]
Phytochemicals[edit]
Plants are an important source of antimicrobial compounds and traditional healers have long used plants to prevent or cure infectious diseases.
[147][148] There is a recent renewed interest into the use of natural products for the identification of new members of the 'antibiotic-ome' (defined as natural products with antibiotic activity), and their application in antibacterial drug discovery in
the genomics era.
[135][149] Phytochemicals are the active biological component of plants and some phytochemicals including
tannins,
alkaloids,
terpenoids and
flavonoids possess antimicrobial activity.
[147][150][151] Some
antioxidant dietary supplements also contain phytochemicals (
polyphenols), such as
grape seed extract, and demonstrate
in vitro anti-bacterial properties.
[152][153][154] Phytochemicals are able to inhibit peptidoglycan synthesis, damage microbial membrane structures, modify bacterial membrane surface hydrophobicity and also modulate quorum-sensing.
[150] With increasing antibiotic resistance in recent years, the potential of new plant-derived antibiotics is under investigation.
[149]
Development of new antibiotics[edit]
In April 2013, the
Infectious Disease Society of America (IDSA) reported that the weak antibiotic pipeline does not match bacteria's increasing ability to develop resistance. Since 2009, only 2 new antibiotics were approved in the United States. The number of new antibiotics approved for marketing per year declines continuously. The report identified seven antibiotics against the
Gram-negative bacilli (GNB) currently in
phase 2 or
phase 3 clinical trials. However, these drugs do not address the entire spectrum of resistance of GNB.
[155][156] Some of these antibiotics are combination of existent treatments:
[citation needed]
Streptomyces research is expected to provide new antibiotics, including treatment against
MRSA and infections resistant to commonly used medication. Efforts of
John Innes Centreand universities in the UK, supported by BBSRC, resulted in the creation of spin-out companies, for example Novacta Biosystems, which has designed the type-b
lantibiotic-based compound NVB302 (in phase 1) to treat
Clostridium difficile infections.
[158][159] Possible improvements include clarification of clinical trial regulations by FDA. Furthermore, appropriate economic incentives could persuade pharmaceutical companies to invest in this endeavor.
[156] In the US, the
Antibiotic Development to Advance Patient Treatment(ADAPT) Act was introduced with the aim of fast tracking the drug development of antibiotics to combat the growing threat of 'superbugs'. Under this Act, FDA can approve antibiotics and antifungals treating life-threatening infections based on smaller clinical trials. The
CDC will monitor the use of antibiotics and the emerging resistance, and publish the data. The FDA antibiotics labeling process, 'Susceptibility Test Interpretive Criteria for Microbial Organisms' or 'breakpoints', will provide accurate data to healthcare professionals.
[160][161]According to Allan Coukell, senior director for health programs at The Pew Charitable Trusts, "By allowing drug developers to rely on smaller datasets, and clarifying FDA's authority to tolerate a higher level of uncertainty fohese drugs when making a risk/benefit calculation, ADAPT would make the clinical trials more feasible."